| General Physics Equipments |
 |
Frank Hertz Experiment |
| |
|
Introduction
From the early spectroscopic work it is clear that atoms emitted radiation at discrete frequencies; from Bohr’s model, the frequency of the radiation n is related to the change of energy levels through DE=hn. It is then to be expected that transfer of energy to atomic electrons by any mechanism should always be in discrete amounts. One such mechanism of energy transfer is through inelastic scattering of low-energy electrons. |
|
| |
|
Frank and Hertz in 1914 set out to verify these considerations.
- It is possible to excite atoms by low energy electron bombardment.
- The energy trasferred from electrons to the atoms always had discrete values.
- The values so obtained for the energy levels were in agreement with spectroscopic results.
Thus the existence of atomic energy levels put forward by Bohr can be proved directly. It is a very important experiment and can be performed in any college or University level laboratory. |
 |
| |
|
Operating Principle |
|
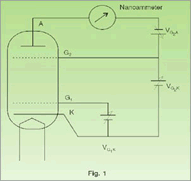
The Frank-Hertz tube in this instrument is a tetrode filled with the vapour of the experimental substance. Fig.1 indicates the basic scheme of experiment. The electrons emitted by filament can be accelarated by the potential VG2K between the cathode and the grid GV2. The grid G2 helps in minimising space charge effects. The grids are wire mesh and allow the electrons to pass through. The plate A is maintained at a potential slightly negative with respect to the grid V2 GV2. This helps in making the dips in the plate current more prominent. In this experiment, the electron current is measured as a function of the voltage VG2K. As the voltage increases, the electron energy goes up and so the electron can overcome the retarding potential VG2A to reach the plate A. This gives rise to a current in the ammeter, which initially increases. As the voltage further increases, the electron energy reaches the threshold value to excite the atom in its first allowed excited state. In doing so, the electrons loose energy and therefore the number of electrons reaching the plate decreases. This decrease is proportional to the number of inelastic collisions that have occured.When the VG2K is increased further and reaches a value twice that of the first excitation potential, it is possible for an electron to excite an atom halfway between the grids, loose all its energy, and then gain anew enough energy to excite a second dip in the current. The advantage of this type of configuration of the potential is that the current dips are much more pronounced, and it is easy to obtain five fold or even larger multiplicity in the excitation of the first level.
|
|
 |
| |
|
| Frank-Hertz Experiment Set-up, Model: FH-3001, consists of the following: |
|
| |
|
|
|
|
The instrument can, not only lead to a plot of the amplitude spectrum curve by means of point by point measurement, but also directly display the amplitude spectrum curve on the oscilloscope screen. This instrument can thus be used as a classroom experiment as well as for demonstration to a group of students. |
|
| |
|
Analysis of Data |
|
Data obtained for the excitation potential point by point are shown in Fig. 3. The readings are taken for 1V changes on grid 2 (VG2K). A significant decrease in electron (collector) current is noticed every time the potential on grid 2 is increased by approximately 12V, thereby indicating that energy is transferred from the beam in (bundles) "quanta" of 12eV only. Indeed, a prominent line in the spectrum of argon exists at 1048Å corresponding to eV=11.83.
The location of the peaks is indicated in Fig. 3. Average value of spacing between peaks is 11.75eV compared with the accepted value of 11.83V. |
|
| |
|
|
|
  |
| |
|
|
|
|


